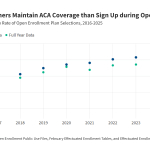
ACA Marketplace Enrollment is Down in 2026—But All of the Data Isn’t in Yet
This brief explains the limitations of early data in understanding the impact of the expiration of enhanced premiums tax credit on ACA enrollment. It also provides a timeline of when more complete data will become available.