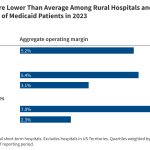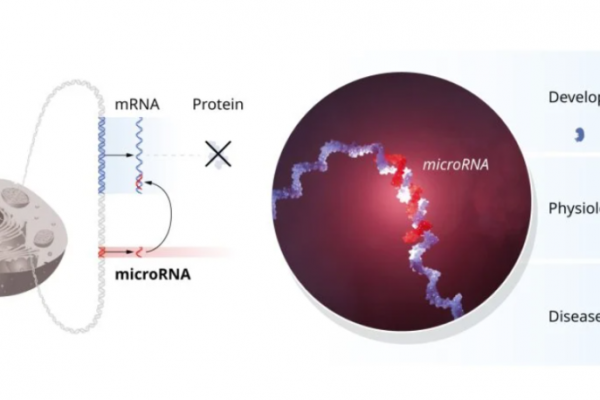
What is microRNA and why did its discovery merit this year’s Nobel Prize for Medicine?
This year, the Nobel Prize in medicine went to Victor Ambros, currently a Professor of Natural Science at the University of Massachusetts Medical School, and Gary Ruvkun, a Professor of Genetics at Harvard Medical School. The two men won the award for their discovery of microRNA. One of the key questions of science is figuring…