Prior authorizations: why they matter and how to navigate the process
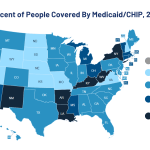
Health payers created the prior authorization system as a cost and quality control strategy, ensuring patients receive only the most necessary, evidence-based, cost-effective and quality care. Nevertheless, real-life implementation has shown that prior authorizations often backfire on their original intents, increasing overall care costs beyond what is necessary. It also delays patient care, contributes to…