
Advantages and Conflicts Surrounding the New ICAN Act
The Improving Care and Access to Nurses (ICAN) Act would cut the red tape for advanced practice nurses to provide care, says AANA’s president

The Improving Care and Access to Nurses (ICAN) Act would cut the red tape for advanced practice nurses to provide care, says AANA’s president

Apellis Pharmaceuticals and Astellas Pharma have the first two FDA-approved therapies for geographic atrophy, but Character Biosciences claims its approach could be best in class for treating this advanced form of dry age-related macular degeneration. The biotech’s Series B round will finance clinical tests of two programs, each addressing a different stage of the disease….

Navina closed a $55 million Series C funding round led by Goldman Sachs. The company, based in Israel and New York, uses AI to help primary care physicians thrive in value-based care models. The post Navina Snags $55M for Its Value-Based Care AI Copilot appeared first on MedCity News.

Following evidence showing that bacterial vaginosis affects both men and women, Wisp has launched a male BV partner treatment. The post Wisp Unveils Male Bacterial Vaginosis Partner Treatment appeared first on MedCity News.
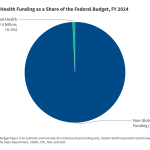
Provides ten key facts about the U.S. global health response, as it was before the Trump administration began.
ProsperityEHR execs discuss the acquisition that is serving as the foundation for their EHR startup in behavioral health

Dr. Monarez is the agency’s current acting director

For all the talk about this problem, the industry has struggled to make big headway. But there are solutions — here are a few steps that make a substantial difference. The post What Healthcare Workers Need Now to Improve Their Wellbeing appeared first on MedCity News.

Deploying a robust continuous improvement methodology is especially beneficial in the earliest phases of drug development. It can help ensure the delivery of high-quality, reliable, timely bioanalytical and clinical data to make informed decisions about either moving on to the next drug development milestone or pivoting to another strategy. The post Continuous Process Improvement: The…

[Sponsored] If you get migraines, you know how important it is to get quick relief. What is the best non-prescription migraine treatment? The post The Best Non-Prescription Migraine Treatment: 5 Tech Devices for Pain Relief appeared first on MedCity News.