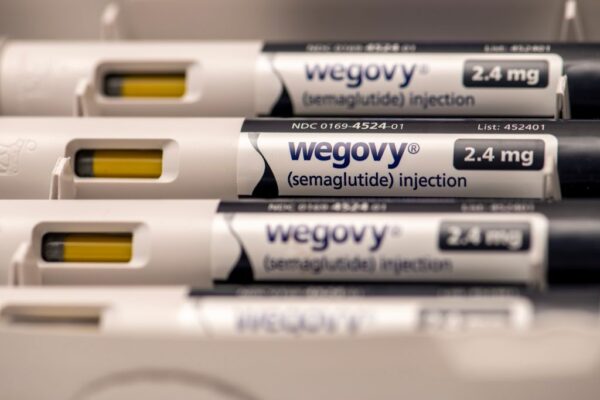
Novo Nordisk’s Wegovy achieved statistically significant improvement in a Phase 3 study in the fatty liver disease MASH. Based on these preliminary results, the company plans to seek U.S. and European Union approvals in this indication in 2025.
The post Novo Nordisk GLP-1 Drug Meets Goals of MASH Trial, Setting Stage for FDA & EMA Filings appeared first on MedCity News.