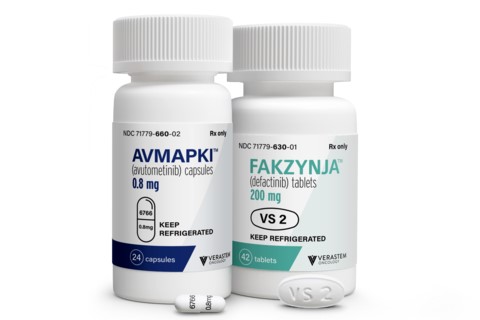
Verastem Oncology developed its drug combination for low-grade serous ovarian cancer (LGSOC) driven by KRAS mutations. As the first treatment specifically approved for this rare cancer, clinicians expect it will become the new standard of care.
The post Accelerated FDA Approval Makes Verastem Drug the First Therapy for Rare Type of Ovarian Cancer appeared first on MedCity News.