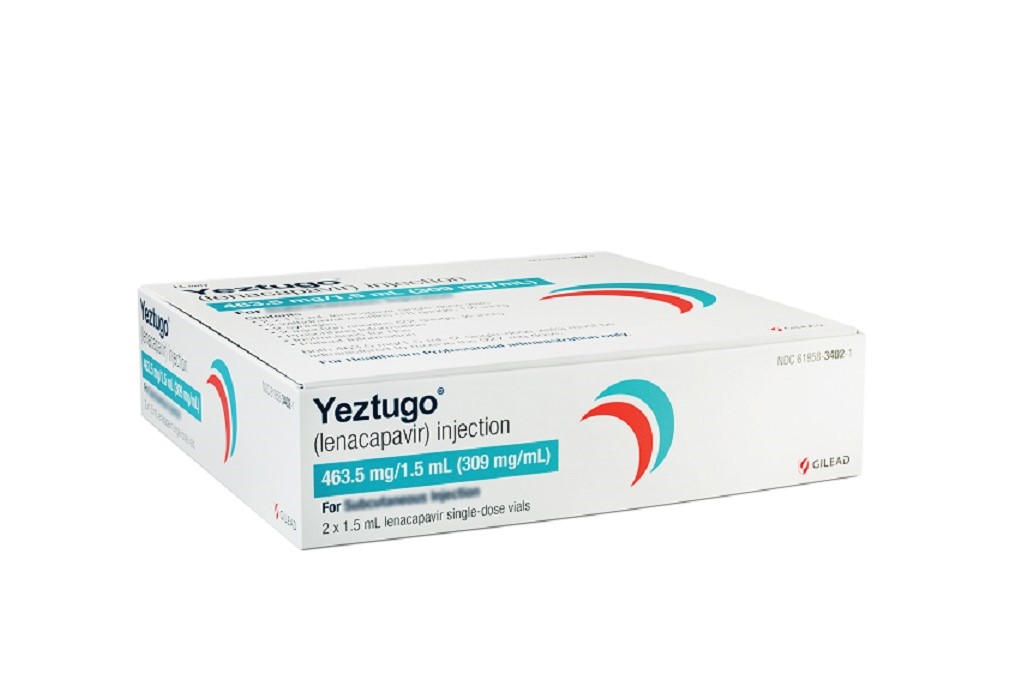
Gilead Sciences drug lenacapavir, brand name Yeztugo, is now approved for HIV pre-exposure prophylaxis. The twice injectable medication is hoped to improve uptake and adherence to PrEP.
The post Gilead Sciences Lands First FDA Approval of a Twice-a-Year HIV PrEP Drug appeared first on MedCity News.