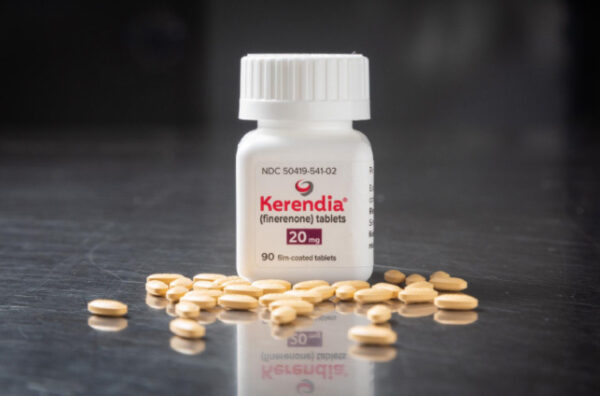
The FDA approved Bayer’s Kerendia for reducing the risk of heart failure in patients with mildly reduced ejection fraction, a measure of how much blood the organ can pump. The daily pill was first approved in 2021 for patients with chronic kidney disease associated with type 2 diabetes.
The post Bayer’s Blockbuster Hopeful Kerendia Expands its FDA Approval to Type of Heart Failure appeared first on MedCity News.