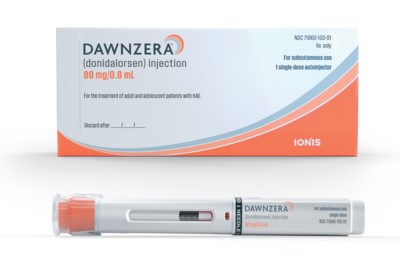
The FDA approved Ionis Pharmaceuticals’ Dawnzera for preventing swelling attacks caused by the rare disease hereditary angioedema. A Takeda Pharmaceutical product dominates this market, but Ionis has clinical data showing patients had better outcomes after switching to Dawnzera from Takeda’s drug and other currently available HAE medications.
The post In Crowded Market for a Rare Disease, Ionis Bets Patients Will Switch to Its New First-in-Class Drug appeared first on MedCity News.