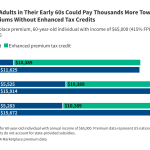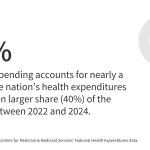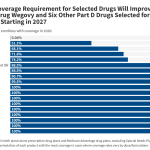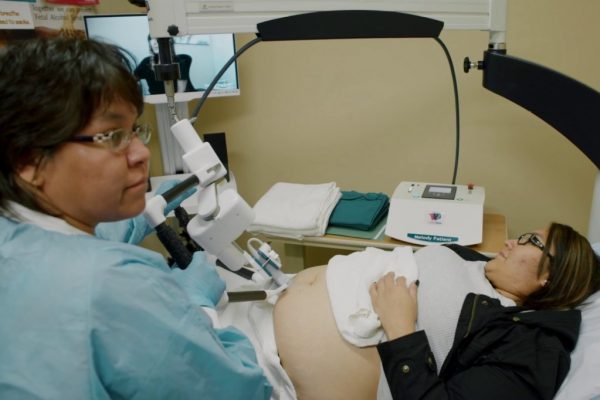
Alabama’s ‘Pretty Cool’ Plan for Robots in Maternity Care Sparks Debate
It sounds like something from a science fiction novel, but Alabama officials’ plan to use robots to improve care for rural pregnant women and their babies is real. During a January White House roundtable touting the first grants to states under a new $50 billion rural health fund, Centers for Medicare & Medicaid Services Administrator…